Company Information
Ask for more detail from the seller
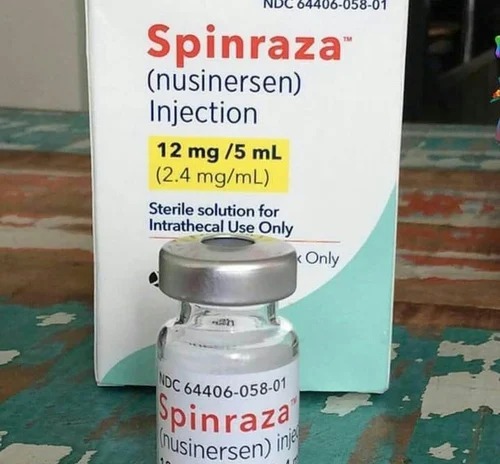
Contact SupplierThe U.S. Food and Drug Administration today approved Spinraza (nusinersen), the first drug approved to treat children and adults with spinal muscular atrophy(SMA), a rare and often fatal genetic disease affecting muscle strength and movement.Biogen, which is licensing Spinraza from Ionis Pharmaceuticals, said this week that one dose will have a list price of $125,000. That means the drug will cost $625,000to $750,000 to cover the five or six doses needed in the first year, and about$375,000 annually after that, to cover the necessary three doses a year